Pfizer Says Vaccine for Younger Children Could Be Ready by Halloween
A smaller dose of the COVID-19 vaccine produced by Pfizer and BioNTech produced a safe and “robust” immune response in children ages 5-11 in a clinical trial, the company said Monday morning.
The smaller, two-dose regimen (it’s about a third of the size of the adult and teen vaccine), administered 21 days apart, had a “favorable safety profile and robust neutralizing antibody responses,” a release from the drugmakers said.
About 43% of children 12-17 are vaccinated, the American Academy of Pediatrics said recently. Pediatric cases of COVID-19 are continuing to rise, most recent data from UT Southwestern shows.
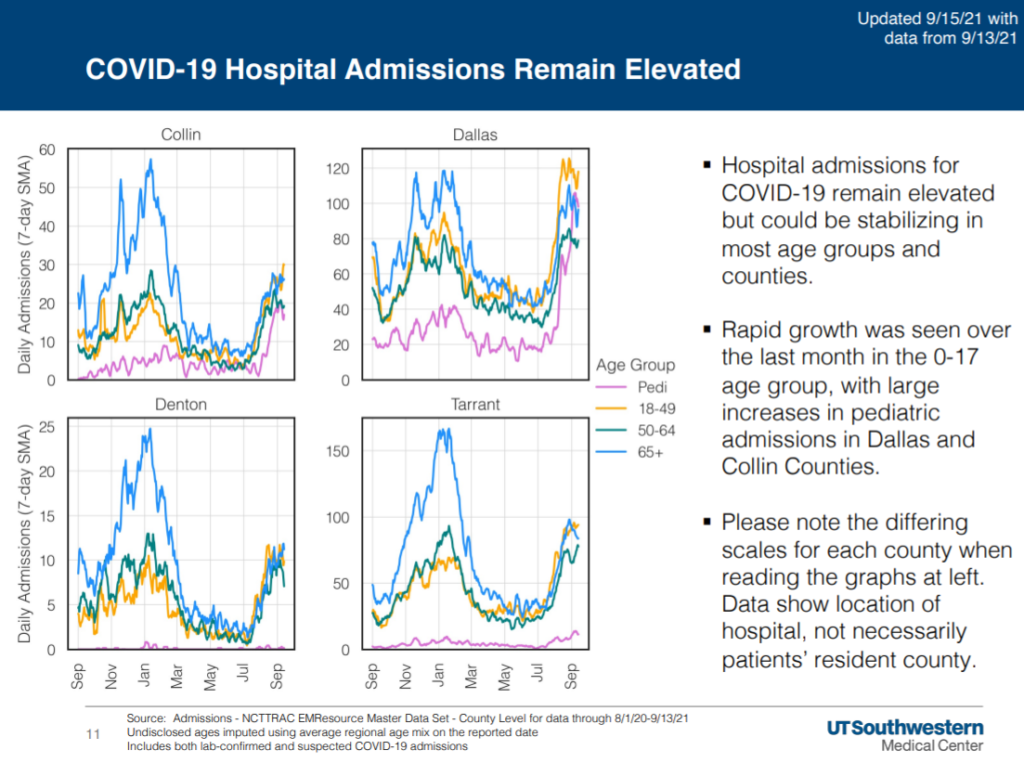
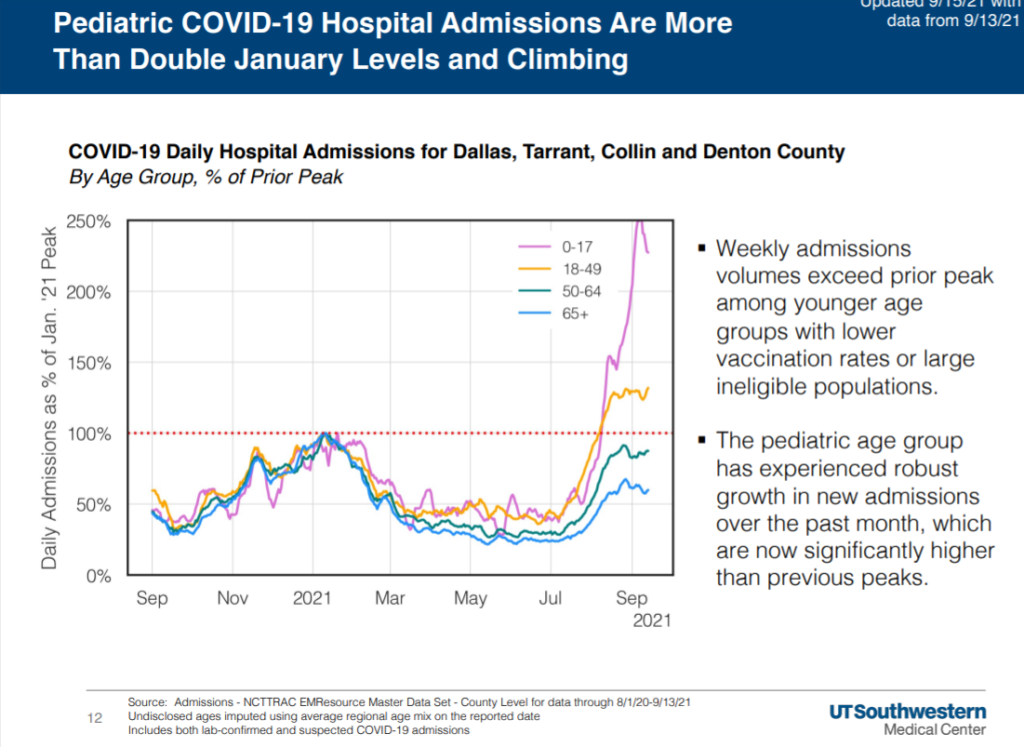
“COVID-19 transmission appears to be slightly reaccelerating in the region, but the percentage of cases resulting in hospital admission is declining, as case growth is primarily seen in those under age 20,” researchers said. “Pediatric hospital admissions are nearly two and a half times January volumes, likely the impact of the return to in-person schooling. Increased hospitalizations reflect the large numbers of individuals who are not yet or cannot yet be vaccinated and are therefore particularly susceptible to infection.”
Pfizer’s clinical trial included more than 2,200 children. The data will be submitted to the Food and Drug Administration and other health agencies possibly as early as the end of September, paving the way for a potential availability before Halloween.
“Over the past nine months, hundreds of millions of people ages 12 and older from around the world have received our COVID-19 vaccine. We are eager to extend the protection afforded by the vaccine to this younger population, subject to regulatory authorization, especially as we track the spread of the Delta variant and the substantial threat it poses to children,” said Albert Bourla, Pfizer CEO and chairman. “Since July, pediatric cases of COVID-19 have risen by about 240 percent in the U.S. – underscoring the public health need for vaccination. These trial results provide a strong foundation for seeking authorization of our vaccine for children 5 to 11 years old, and we plan to submit them to the FDA and other regulators with urgency.”
The companies said in a joint press release that the shots were well-tolerated by the children in the trial, and produced results comparable to those in a study of people ages 16-25.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” said Dr. Ugur Sahin, CEO and co-founder of BioNTech. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”