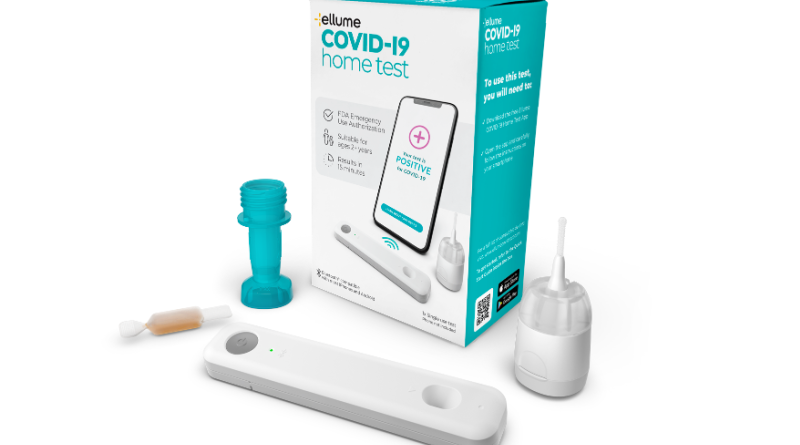
New At-Home COVID-19 Test Authorized by FDA
As we all head into the final stretch before the Christmas holiday, good news about the fight against COVID-19 is tempered with the knowledge that to be safe, the holidays will need to look a little different this year. Here are today’s main bullet points:
- New at-home COVID-19 test authorized by FDA;
- Dallas County reports 1,947 new COVID-19 cases.
New At-Home COVID-19 Test Authorized by FDA
The Food and Drug Administration announced Tuesday that it approved a new at-home COVID-19 test that consumers can easily purchase without a prescription, administer themselves, and then get results in as quickly as 15 minutes.
“Today’s authorization is a major milestone in diagnostic testing for COVID-19,” FDA Commissioner Stephen Hahn said in a statement announcing the authorization.
The test will cost around $30, the manufacturer, Ellume, said. It will be available by next month.
The new test isn’t the first at-home test – others have been approved prior to this week. But it is the first at-home test that doesn’t require a prescription, or that the sample be sent to a lab.
The new test requires users to use a special swab to collect a sample from just inside their nose, and can be used on adults and children as young as 2. After collecting the swab, the user then ads a few drops of liquid to the sample, places it on a small plastic device, and results are transmitted to a smartphone app.
“In data submitted to the FDA from an independently run, simulated home-setting clinical study of 198 subjects ranging in age from 2 years to 82 years, the Ellume COVID-19 Home Test demonstrated 96% accuracy, with an overall sensitivity of 95% (positive percent agreement) and specificity of 97% (negative percent agreement) when compared to an emergency use-authorized RT-PCR laboratory test,” the company’s announcement said. “In individuals presenting with COVID-19 symptoms, the Ellume COVID-19 Home Test demonstrated a sensitivity of 96% and specificity of 100%, and in asymptomatic individuals, the test demonstrated a sensitivity of 91% and specificity of 96%.”
Dallas County Reports 1,947 New COVID-19 Cases Tuesday
Dallas county health officials reported 1,947 new cases of COVID-19 Tuesday, with 1,549 confirmed and 398 probable.
The county also reported six new deaths attributed to COVID-19. Among the dead are a Dallas woman in her 40s, two Dallas men in their 50s, a Dallas woman in her 60s, a Dallas woman in her 70s, and a Dallas woman in her 80s. All had underlying high-risk health conditions.
Over the past 30 days, there have been 4,520 COVID-19 cases in school-aged children and staff reported from 735 separate K-12 schools in Dallas County, including 681 staff members. Of these cases, 534 have been associated with extracurricular activities, including athletics.
There are 97 active long-term care facility outbreaks. Over the past 30 days, a total of 928 COVID-19 cases have been reported from these facilities, including 364 staff members. Of these cases, 35 have been hospitalized, and 41 have died, including two deaths of staff members. Twenty-six outbreaks of COVID-19 in congregate-living facilities like homeless shelters and group homes have been reported in the past 30 days associated with 166 cases, including 8 hospitalizations. One facility has reported 89 COVID-19 outbreak cases since October.
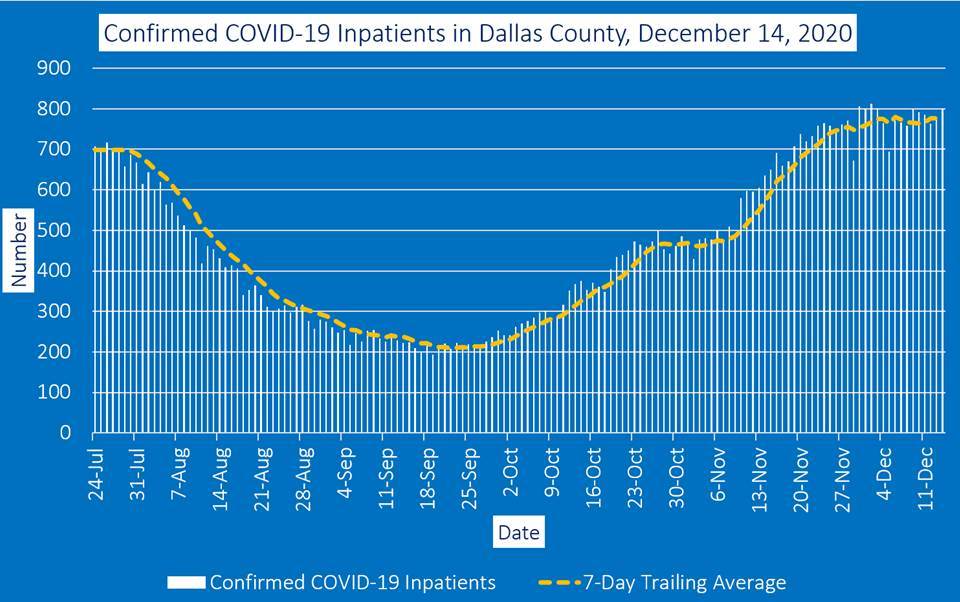
The county said that the number of COVID-19 hospitalizations Monday was 800 patients. Emergency room visits for COVID-19 symptoms represented about 24% of all ER visits, according to information reported to the North Central Texas Trauma Regional Advisory Council.
“Today we add 1,947 new cases and six deaths to our numbers for COVID. This also marks the first day of the COVID vaccine at Parkland Hospital, and in the coming days, more and more hospitals will get the vaccine, and will be able to give them to their healthcare heroes,” said Dallas County Judge Clay Jenkins. “In the coming days, we’ll begin the vaccinations in nursing homes, and as more vaccines are approved and production ramps up, more supply will become available.
“It will still be some time before the general public will have broad access to the vaccines with the government working to get it to people in the order of their degree of risk,” he added. “We must all use the tools available to us during this time of high spread and throughout this holiday season and New Year until the vaccine is widely administered and able to do its effect.”
Those tools, he said, included wearing masks, limiting interactions with people you don’t live with, and practicing social distancing.