Slowing COVID-19 Vaccination Rate Slows Reaching ‘Herd Immunity’
A continued slowing vaccination rate recently pushed the Parkland Center for Clinical Innovation (PCCI) to push back its forecast for when Dallas County could reach herd immunity to late July.
Herd immunity is the indirect protection from an infectious disease that happens when a population is immune either through vaccination or immunity developed through previous infection,” the World Health Organization explains.
The new herd immunity analysis includes the up-to-date vaccination percentages for Dallas County as well as a change in the percentage of residents confirmed and presumed to have been infected. PCCI says these factors indicate that the county’s herd immunity rate is now 74% of the total population.
“The most critical factor in the fight to crush COVID remains vaccinations for the whole population, particularly with the county’s younger, working population and the recently approved high school and middle school students,” said PCCI’s president and CEO Steve Miff. “Convenience, missed work or school from vaccine side-effects, and concerns about providing identifications for the transient and un-documented populations remain as key barriers to vaccinations and continue to be a key decision driver for the 20-30 percent of the unvaccinated population who are not opposed to receiving a vaccine, but have not yet rolled up their sleeves.”
A new UT Southwestern virologic study indicates a ratio of 1:4 for confirmed positive (tested and confirmed) to presumed infected (positive antibodies, but never tested) population for Dallas County.
With this information, PCCI has updated the presumed infected adult population from a 1:3 ratio to the 1:4 ratio. This increase in the presumed infected results also in adjustment of the vaccinated population of Dallas estimated to have had prior COVID-19 infection and recovered from 21 percent to 28%, according to PCCI.
The weekly average for vaccinations is slowing below 15,000 first doses per week despite new age group approval for 12-15-year-olds, according to PCCI data.
As of May 20, 1,018,696 people in Dallas County were vaccinated, with 798,775 being fully vaccinated. Experts say this indicates that about 40% of the whole county population, 52% of the over 18-year-old population, and 77% of the over 65-year-old have received at least one dose of the vaccine.
As of May 20, 26 Dallas County ZIP codes are estimated to have the 80 percent threshold.
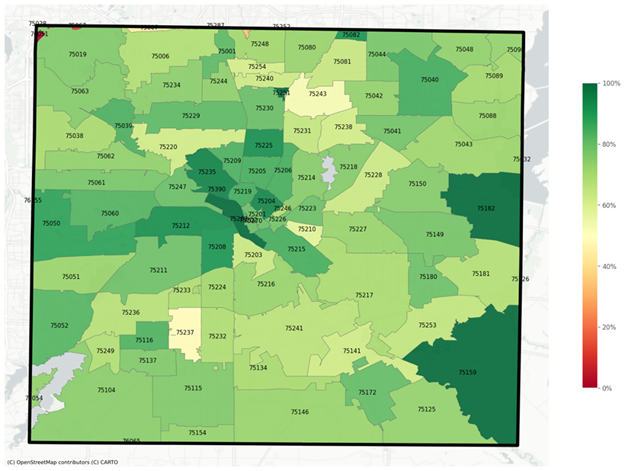
“With this new analysis, we are able to pinpoint where the least vaccinated populations are in the county,” said Miff. “Our public health leaders are taking action and making progress toward reaching those areas, but each of us can take responsibility to quickly receive our vaccine and encourage our friends and neighbors to do the same, especially for those who live in neighborhoods with the low vaccination rates. Vaccines are the fastest and safest way we can get back to any sort of normal.”
For areas already at or above 80%, and for the rest of the county, until vaccination rates increase, experts say there will likely continue to be low levels of infections, hospitalizations, and deaths.
“The virus will continue to linger in the background at relatively low and steady rates, with the periodic flareup. The best thing we can do is continue to vaccinate to reach and surpass the 80 percent vaccination mark and then drive both personal and community protection through the vaccines,” said Dr. George ‘Holt’ Oliver, vice president of clinical Informatics at PCCI.
In other news:
- Dallas County will shut down the mass COVID-19 vaccination site at Fair Park after August 1 because of the end of the county’s contract to use the site and to allow for preparation for the State Fair of Texas, the Dallas Morning News reports.
- UT Southwestern has a new post-COVID clinic designed to help those with ‘long haul’ symptoms after COVID-19 infection, WFAA reports.
- Moderna recently announced its COVID-19 vaccine was ‘highly effective’ at preventing COVID-19 in adolescents as young as 12 in a study and the company plans to submit the results for FDA approval in early June.